

May is Brain Cancer and Brain Tumor Awareness Month (BTAM), a time to raise awareness about brain tumors and educate the community.
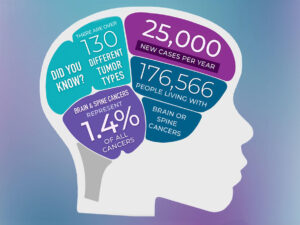
Doctors will diagnose cancers of the brain or central nervous system in about 25,400 people in the United States in 2024, according to the National Cancer Institute. All brain and spine tumors, collectively called central nervous system (CNS) tumors cover over 130 different CNS tumor types. These cancers make up a portion of the more than 94,000 brain tumors alone (including benign tumors) that will occur in this country in 2024.
It can be hard for people with CNS tumors to find accurate information, specialized support, and expert care. You can help by spreading awareness and sharing educational materials like through blogs live striveforgoodhealth.com and other sites in the internet.
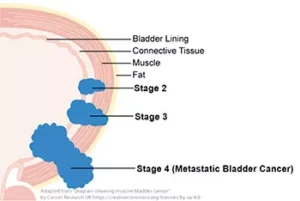
There are many types of brain and spinal cord tumors. The tumors result from the abnormal growth of cells and may be either benign or malignant. Benign brain and spinal cord tumors grow and press on nearby areas of the brain. Normally, they rarely spread into other tissues; the brain tumors that are diagnosed malignant rapidly spread only in brain tissue and remember when your a fetus the brain develops that the spinal cord grows out of made of brain tissue so spreading can go in those 2 areas. A brain tumor malignant can form in the brain or other parts of the central nervous system (CNS), being the spine or cranial nerves. So remember, Malignant tumors in the brain and spinal cord only grow quickly spreading only into the brain and (CNS) spinal cord tissue. The positive note is the tumor stays in those areas but unfortunately it spreads rapidly for most brain tumors. Survival in a brain tumor especially malignant is a survival rate of 5 years or less but there are those cases that have lasted longer but on average its 5 years or less and this would include a benign tumor not operable but it is suppose to grow slower than a malignant tumor. Malignant brain tumors need to be treated as soon as possible to prolong life.
Tumor grading:
Tumor grade has long been a way to define the aggressiveness of a tumor, particularly for malignant brain tumors such as glioma but also for non-malignant (benign) brain tumors including meningioma.
Traditionally, tumors have been classified as grade 1 to 4 based on histology (cells as viewed under a microscope) and molecular markers. Grade 1 tumors occur primarily in children and represent a type separate from grade 2-4 (seen primarily in adults). Grade 2 tumors are considered low grade, but some can be aggressive. Grade 3 and 4 tumors are defined as high grade.
What are molecular markers?
Not all brain tumors are the same. Some tumors have differences in the genetic or molecular makeup of the cells. These differences are called molecular markers, or biomarkers. Molecular markers are becoming increasingly important for brain tumor diagnosis and treatment. For example, some molecular markers help determine how aggressive a tumor may be. Others determine how responsive a tumor will be to treatment.
Some common molecular markers include the following:
- IDH1 and IDH2
- MGMT
- 1p/19q co-deletion
- BRAF
- EGFR
- TP53
- ATRX
- TERT
- PTEN
- NTRK
- FGFR
In 2016, the World Health Organization (WHO) included two molecular markers into the CNS tumor classification system that improved accuracy of glioma diagnosis. In 2021 WHO again updated CNS tumor classification, incorporating new knowledge gained from additional molecular markers and new diagnostic techniques. Tumors are now listed as “CNS grade 1-4” with presence or absence of IDH mutation, a key factor in glioma classification.
Basis Review of Brain & the CNS with how it functions:
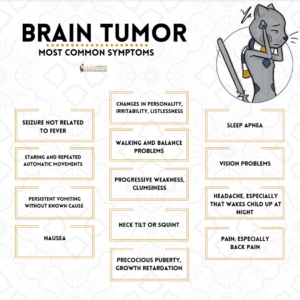
The brain and spinal cord together form the central nervous system (CNS), like we said in knowing this the brain is a complex organ made up of nerves and connective tissue. Nerves in the brain and spinal cord transmit messages throughout the body. The CNS directs and regulates all of the body’s functions. The brain tumor can definitely mess up a lot of these functions depending on where the tumor is located since the brain is broken up in lobes to do different functions that is what causes the wide signs and symptoms of dysfunctions that occur in time with a brain tumor especially that is metastatic.
The CNS is the core of our existence. It controls:
> Personality: thoughts, memory, intelligence, speech, understanding and emotions
> Senses: vision, hearing, taste, smell and touch
> Basic body functions: breathing, heartbeat and blood pressure
> How we function in our environment: movement, balance and coordination
The brain is made up of multiple parts, and each part of the brain is responsible for different body functions. Therefore, brain tumor symptoms, and potential treatment options, depend a great deal on where the tumor is located.
Learning about the normal workings of the brain and spine will help you understand the symptoms of brain tumors, how they are diagnosed and how they are treated.
Major parts of the brain: There are three major parts of the brain:
1. Cerebrum: uses information from senses to tell our body how to respond. It controls reading, thinking, learning, movement, speech, vision, personality and emotions.
2. Cerebellum: controls balance for standing, walking and other motion.
3. Brain stem: connects the brain with the spinal cord and controls basic body functions such as breathing, sleeping, body temperature and blood pressure.
Lobes of the brain
Different lobes of the brain control different functions. The frontal lobe of the brain helps you think and reason. The temporal lobe contains the neural pathways for hearing and vision, as well as behavior and emotions. Having a tumor, or treatment, in one of these lobes could affect the lobe’s specific functions. Additionally, since the brain has areas that connect, it is possible for a brain tumor to impact a function of the brain where the tumor is not specifically located.
Other common brain tumor locations include the meninges (a layer of tissue that covers the brain and spinal cord), skull base (the bottom of the skull), spinal cord, pituitary tumor, and cranial nerves.
Brain tumor statistics:
Brain tumors are reported in people of all ages, races, ethnicities, and genders. Over 1.3 million Americans are living with a primary or secondary/metastatic brain tumor today. Primary tumors originate in the brain, and the most common types are meningiomas, pituitary tumors, and gliomas. Metastatic, or secondary brain tumors arise from outside the brain in another organ such as the breast or lung and spread to other areas of the brain. These are the most common brain tumors.
Unless otherwise specified, the follow statistics come from the Central Brain Tumor Registry of the United States Annual Report:
- Approximately 90,000 people are diagnosed with a primary brain tumor every year.
- Brain and other CNS tumors are the fifth most common cancer.
- Over 1 million people are living with a diagnosis of a primary brain tumor.
- There are more than 100 different types of primary brain and CNS tumors.
- Nearly one-third (27.9 percent) of brain and central nervous system (CNS) tumors are malignant.
- Brain and CNS tumors are the most common cancer diagnosed in children aged 0-14.
- More than 28,000 children in the United States are currently diagnosed with a brain tumor.
- Approximately 3,400 children (aged 0-14) are diagnosed with a primary brain tumor each year.
- Approximately 12,800 adolescents and young adults (aged 15-39) are diagnosed with a primary brain tumor each year.
- The incidence rate for brain and CNS tumors is highest among those aged 85 years and older.
- Each year, approximately 17,200 people die from a malignant brain tumor. Survival after diagnosis with a primary brain tumor varies significantly by age, race, geographical location, tumor type, tumor location, and molecular markers.
Risk Factors for Brain Tumors:
Genetic and hereditary risk factors
Inherited traits are carried in genes. Each individual has two copies of each gene, one from each parent. Genes often contain small changes. Sometimes these changes do not cause any problems, but sometimes these changes are more serious and can interfere with the way the gene is supposed to work.
There are a few rare, inherited genetic syndromes that are associated with brain tumors., including Neurofibromatosis 1 (NF1 gene), Neurofibromatosis 2 (NF2 gene), Turcot syndrome (APC gene), Gorlin syndrome (PTCH gene), Tuberous Sclerosis (TSC1 and TSC2 genes) and Li-Fraumeni syndrome (TP53 gene).
Although 5-10% of persons with brain tumors have a family history of a brain tumor, the vast majority of CNS tumors appear not to be a part of inherited genetic syndromes. A number of studies have identified genetic variants that may be associated with an increased risk of certain brain tumors including glioma and meningioma. Study results from 2017 show that while there are some hereditary similarities in glioma tumors between family members, there is not a statistically significant difference between families having tumors with similar hereditary features as compared to families with tumors having different hereditary features. Also, in families with more than one glioma, the tumors tend to have the same molecular markers. This study continues to collect and analyze data.
Environmental risk factors:
Other than family history, the most consistently identified risk factor associated with brain tumor development is therapeutic or high-dose ionizing radiation. With regard to medical diagnostic radiation exposure, small increases in brain tumor risks have been reported. Although certain brain scans and radiation therapy used to treat brain tumors use ionizing radiation, the risk of developing a new brain tumor due to these causes is very low. Occupational exposures among medical radiation workers have been associated with approximately twice the risk of brain cancer mortality, though data on the level of radiation exposure were not available.
With respect to the impact of non-ionizing radiation from cell phones, the association between this exposure and brain cancer has been the subject of much research. Radio frequency fields were classified by the World Health Organization’s International Agency for Research on Cancer in 2011 as a possible carcinogen following the observation of increased glioma risk among heavy cell phone users: the topic remains under study at present.
Industrial chemicals have long been suspected as a cause of glioma due to their ability to cross the blood–brain barrier. The blood-brain barrier that the human brain has protects the brain from toxins and pathogens. Despite numerous chemical, environmental, and occupational exposures having been explored in epidemiological studies of glioma, results have been inconsistent for most factors. Although not precisely defined, an association between exogenous hormones (e.g., oral contraceptives, hormone replacement therapies) and meningioma risk is often reported and thus patients might discuss this topic with their health care providers.