The WHO-World Health Org. states the following
( News 12/09/2022):
“Getting vaccinated could save your life. COVID-19 vaccines have saved millions of lives since their introduction and provide strong protection against serious illness, hospitalization and death.
It is still possible to get COVID-19 and spread it to others after being vaccinated. Therefore, consider continuing to practice protective and preventive behaviours such as keeping a distance, wearing a mask in crowded and poorly ventilated spaces, practicing hand hygiene, respiratory etiquette (covering your mouth and nose with a bent elbow or a tissue when you cough or sneeze), getting vaccinated and staying up to date with booster doses. However, if you do get COVID-19 after vaccination, you are more likely to have mild or no symptoms than if you hadn’t been vaccinated.
Even if you have had COVID-19, WHO still recommends that you get vaccinated after infection because vaccination enhances your protection against severe outcomes of future COVID-19 infection, and you may be protected for longer. Furthermore, hybrid immunity resulting from vaccine and infection may provide superior protection against existing variants of concern.
To ensure optimal protection, is important to receive COVID-19 vaccine doses and boosters recommended to you by your health authority.
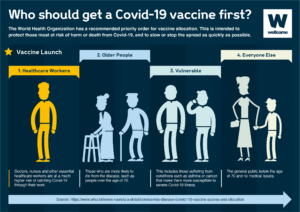
The high-priority group should be prioritized for the primary series vaccines as well as first and additional booster doses. The additional boosters should be administered either 6 or 12 months (depending on your risk category) after the last dose, with the timeframe depending on factors such as age and immunocompromising conditions.
The medium-priority group includes healthy adults – usually under the age of 50–60 without comorbidities, and children and adolescents with comorbidities. SAGE recommends primary series and first booster doses for the medium-priority group. Although additional boosters are safe for this group, SAGE does not routinely recommend them, given the comparatively low public health returns.
The low-priority group includes healthy children and adolescents aged 6 months to 17 years. Primary and booster doses are safe and effective in children and adolescents. However, considering the low burden of disease, SAGE urges countries considering vaccination of this age group to base their decisions on contextual factors, such as the disease burden, cost-effectiveness, and other health or programmatic priorities and opportunity costs.”
So after all this information with all these Vaccines this gives 2 questions?
1-With the new ones now available, WHICH ONE IS BETTER?
2-Which immunity is better: Disease-induced immunity (you had covid and were induced with immunity) or vaccination-induced (immunity by the vaccine itself)?
It turns out it’s not in either situation. Limitations exist with gaining immunity either way – BUT in by getting infected by the virus (disease induced) and by getting vaccinated.
Previous research indicated that disease-induced immunity wasn’t necessarily better and that vaccines created more effective and longer-lasting immunity than natural immunity. Disease-induced immunity, specifically, can be spotty and appears to be somewhat related to how severe the illness was (more severely ill persons appear to have a greater immune response than those with very mild illness or asymptomatic infection). Some people may have a good antibody response, while others don’t get much of any response.
The additional benefit of vaccine-induced immunity? Fewer downsides.
- Both immunity types start to wane within 60 to 90 days, depending on how your body reacts
- Getting the virus comes with more risks, including the potential to develop severe illness, long-COVID or death
Vaccination helps protect against the most serious risks. “Yes, the vaccine has a few rare serious adverse events associated with it,” says infectious diseases expert Mark Rupp, MD. “However, the risk of adverse events is much lower than the substantial risks of serious infection and the risk of long-COVID that you get with the disease.”
Studies now show that both types of immunity are beneficial.
“Recent data analyses indicate that disease-induced immunity can be as long-lasting or even longer-lasting in some instances than vaccine-induced immunity,” adds Dr. Rupp. “Both appear to do a pretty good job protecting from severe illness and death.”
The best protection against severe outcomes: Hybrid immunity
Hybrid immunity = natural immunity + vaccination
According to an analysis published in The Lancet Infectious Diseases, a recent, robust study shows that hybrid immunity is longer lasting and more effective than disease-induced immunity or vaccination alone.
Which immunity is better: Disease-induced immunity or vaccination-induced immunity?
It turns out it’s not an either-or situation. Limitations exist with gaining immunity either way – by getting infected by the virus (disease induced) and by getting vaccinated.
Previous research indicated that disease-induced immunity wasn’t necessarily better and that vaccines created more effective and longer-lasting immunity than natural immunity. Disease-induced immunity, specifically, can be spotty and appears to be somewhat related to how severe the illness was (more severely ill persons appear to have a greater immune response than those with very mild illness or asymptomatic infection). Some people may have a good antibody response, while others don’t get much of any response.
The additional benefit of vaccine-induced immunity? Fewer downsides.
- Both immunity types start to wane within 60 to 90 days, depending on how your body reacts
- Getting the virus comes with more risks, including the potential to develop severe illness, long-COVID or death
Vaccination helps protect against the most serious risks. “Yes, the vaccine has a few rare serious adverse events associated with it,” says infectious diseases expert Mark Rupp, MD. “However, the risk of adverse events is much lower than the substantial risks of serious infection and the risk of long-COVID that you get with the disease.”
Studies now show that both types of immunity are beneficial.
“Recent data analyses- indicate that disease-induced immunity can be as long-lasting or even longer-lasting in some instances than vaccine-induced immunity,” adds Dr. Rupp. “Both appear to do a pretty good job protecting from severe illness and death.”
The best protection against severe outcomes: Hybrid immunity
Hybrid immunity = natural immunity + vaccination
According to an analysis published in The Lancet Infectious Diseases, a recent, robust study shows that hybrid immunity is longer lasting and more effective than disease-induced immunity or vaccination alone.
THE WHO further states (News 12/09/2022):
“EMA’s human medicines committee (CHMP) has recommended authorising an adapted bivalent vaccine targeting the Omicron subvariants BA.4 and BA.5 in addition to the original strain of SARS-CoV-2. This recommendation will further extend the arsenal of available vaccines to protect people against COVID-19 as the pandemic continues and new waves of infections are anticipated in the cold season.”
First B-4 and B-5 or known also as 4-5 COVID-19 vaccines) for use as a booster dose in individuals aged 12 years and older. The new bivalent vaccine comprises 15 micrograms of famtozinameran based on the Omicron variants BA. 4 and BA. 5, and 15 micrograms of tozinameran based on the original strain of SARS CoV-2.
Through the European Medicines Agency (https://www.ema.europa.eu/en/news/adapted-vaccine-targeting-ba4-ba5-omicron-variants-original-sars-cov-2-recommended-approval) they state the following:
“EMA’s human medicines committee (CHMP) has recommended authorising an adapted bivalent vaccine targeting the Omicron subvariants BA.4 and BA.5 in addition to the original strain of SARS-CoV-2. This recommendation will further extend the arsenal of available vaccines to protect people against COVID-19 as the pandemic continues and new waves of infections are anticipated in the cold season.
Comirnaty Original/Omicron BA.4-5 is for use in people aged 12 years and above who have received at least a primary course of vaccination against COVID-19. This vaccine is an adapted version of the mRNA COVID-19 vaccine Comirnaty (Pfizer/BioNTech).
Vaccines are adapted to better match the circulating variants of SARS-CoV-2 and are expected to provide broader protection against different variants. Prompt assessment of the available data on these adapted vaccines will enable their timely deployment in the autumn vaccination campaigns.
In its decision to recommend the authorisation of Comirnaty Original/Omicron BA.4-5, the CHMP took into account all the available data on Comirnaty and its adapted vaccines, including the recently authorised adapted vaccine Comirnaty Original/Omicron BA.1 as well as investigational vaccines against other variants of concern.
The CHMP based its opinion in particular on the clinical data available with Comirnaty Original/Omicron BA.1. Apart from containing mRNA matching different, but closely related, Omicron subvariants, Comirnaty Original/Omicron BA.4-5 and Comirnaty Original/Omicron BA.1 have the same composition. Clinical studies with Comirnaty Original/Omicron BA.1 showed that the vaccine was more effective at triggering an immune response against the BA.1 subvariant than Comirnaty, and was as effective as Comirnaty against the original strain. Side effects were comparable to those seen with Comirnaty. This was further supported by data from investigational vaccines targeting other variants which have also shown similar safety profiles and predictable immune responses against the strains they target.
The CHMP’s opinion for Comirnaty Original/Omicron BA.4-5 is also based on data on its quality and manufacturing process, which confirmed that it meets the EU standards for quality. In addition, immunogenicity data (the ability of the vaccine to trigger an immune response) from laboratory (non-clinical) studies provided supportive evidence that Comirnaty Original/Omicron BA.4-5 triggers adequate immunity against the strains it targets.
Based on all these data, the CHMP concluded that Comirnaty Original/Omicron BA.4-5 is expected to be more effective than Comirnaty at triggering an immune response against the BA.4 and BA.5 subvariants. The vaccine’s safety profile is expected to be comparable to that of Comirnaty Original/Omicron BA.1, and of Comirnaty itself for which a large amount of data is available.”