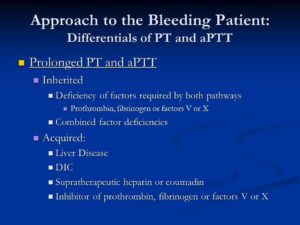
Prolonged PT and aPTT. Inherited. Deficiency of factors required by both pathways. Prothrombin, fibrinogen or factors V or X. Combined factor deficiencies. Acquired: Liver Disease. DIC. Supratherapeutic heparin or coumadin. Inhibitor of prothrombin, fibrinogen or factors V or X.
Prothrombin deficiency is a bleeding disorder that slows the blood clotting process. People with this condition often experience prolonged bleeding following an injury, surgery, or having a tooth pulled. … Women with prothrombin deficiency can have prolonged and sometimes abnormally heavy menstrual bleeding. This blood clotting factor II deficiency. Remember we have blood clotting factors that have to be present for the blood clotting factor but if one is lacking then bleeding can occur.
Practice Essentials
Clotting factor II, or prothrombin, is a vitamin K–dependent proenzyme that functions in the blood coagulation cascade. Factor II deficiency is a rare, inherited or acquired bleeding disorder disorder with an estimated incidence of one case per 2 million population. [1]
Inherited factor II deficiency is an autosomal recessive disorder that can manifest as hypoprothrombinemia, a decrease in the overall synthesis of prothrombin; or as dysprothrombinemia, the synthesis of dysfunctional prothrombin. [2, 3, 4] Homozygous individuals are generally asymptomatic and have functional prothrombin levels of 2-25%. However, symptomatic individuals may experience one or more of the following [1] :
-
Easy bruising
-
Epistaxis
-
Soft-tissue hemorrhage
-
Excessive postoperative bleeding
-
Menorrhagia
-
Muscle hematomas
-
Hemarthrosis
-
Intracranial bleeding
In true hypoprothrombinemia, immunologic assays correlate well with functional assays in that both reveal low prothrombin values. Heterozygous patients are generally asymptomatic and have prothrombin levels of 50% or greater on both immunologic and functional assays.
In dysprothrombinemia, only the functional assay for prothrombin returns significantly reduced values, whereas the immunologic assay reveals normal values. Acquired factor II deficiency can be caused by severe liver disease, vitamin K deficiency, anticoagulant drugs (eg, warfarin), or the presence of an antibody directed against the protein. [5]
Aside from the prothrombin deficiencies, another disorder of prothrombin is the prothrombin 20210a mutation. First reported in 1996 as a familial cause of venous thromboembolism, the prothrombin 20210a mutation results in increased levels of plasma prothrombin and a concurrent increased risk for the development of thrombosis. [6]
Prothrombin 20210a has an estimated prevalence of 2% in whites. [7, 8] The mutation is more prevalent in those of southern European descent than in those of northern European descent, and it is rarely seen in Asians or Africans. [7] A study of patients in Turkey revealed the presence of the prothrombin 20210a mutation in 0.7% of subjects. [9]
Individuals carrying the prothrombin 20210a mutation have a 2- to 3-fold increased risk for developing thrombosis. [6, 10] One case-control study found evidence of an increased risk of developing an ischemic cerebrovascular event in men aged younger than 60 years with the prothrombin 20210a mutation. [11] A study of cancer patients in the Netherlands found that the presence of the prothrombin 20210a mutation in these patients may increase the risk of venous thrombosis to a level greater than that attributable to the malignancy alone. [12]
The prothrombin 20210a mutation can be identified without DNA analysis and should be considered in any patient experiencing a thrombotic event without other risk factors. Treatment with oral anticoagulants is useful in preventing recurrence in patients with the mutation who have already experienced a thrombotic event. Additionally, women who are known to carry the mutation may want to avoid oral contraceptives because of the additional risk of thrombosis.
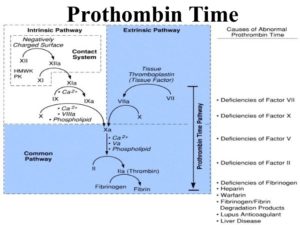
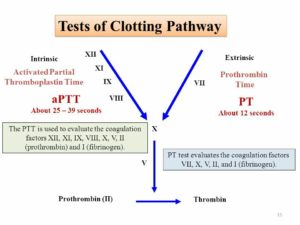
Laboratory studies for factor II deficiency include coagulation studies and clotting factor assays (see Workup). Coagulation study results are as follows:
-
Prothrombin time (PT) is prolonged
-
Activated partial thromboplastin time (aPTT) is prolonged
-
Bleeding time is within reference range
Treatment of factor II deficiency is aimed at restoring circulating factor II to levels sufficient for hemostasis. Levels greater than 30% of normal are usually adequate. Treatment measures include fresh frozen plasma (FFP), prothrombin complex concentrates (PCCs), and vitamin K. Additionally, in patients with acquired factor II deficiency, the underlying cause should be found and treated.
Frequency
Both congenital and acquired factor II deficiencies are rare. The prevalence of congenital factor II deficiency is approximately 1 per 1 to 2 million population. [41]
Mortality/Morbidity
Congenital factor II deficiency is a lifelong bleeding disorder. Death can result because of massive hemorrhage from relatively minor accidents or trauma. Hemorrhage can also occur as a result of surgery if precautions are not taken. Intracranial bleeding is another serious sequela of this disorder. Rarely, hemarthroses can occur. [2]
Myocardial infarction is a rare complication in young people, with coronary thrombosis due to hypercoagulable states being one cause. A heterozygote prothrombin gene mutation (G-20210-A) and protein S deficiency were described in a 19-year-old with a myocardial infarction with normal coronary arteries. [42]
Race- sex-, and age-related demographics
Factor II deficiency has no known racial or ethnic predilection. Males and females are affected equally. Patients with severe congenital factor II deficiency present early in life, whereas those with less severe forms can present at any age. Acquired forms can be observed in all age groups.