Archive | May 2017
Part V Other barriers in prevention of Infections in hospitals & long care facilities!
Personal Protective Equipment
Healthcare professionals are both barriers to and carriers of infection. To avoid distributing infection among patients, healthcare professionals must remain diligent in the use of personal protective equipment, or PPE. Gloves, gowns, shoe covers, caps, masks, respirators, hair covers, face protection, and eye protection are all vital in preventing the spread of infection. Nurses who use PPE effectively help prevent the spread of infection through the touch of a hand, through contact with their uniform, and through air and droplets from their respiratory tract. In turn, they are personally protected against any organisms the patient may carry.
Gloves are the most frequently worn item of PPE. Gloves reduce the opportunity for microbes on healthcare workers’ hands to be spread to patients during care or invasive procedures. Removing gloves between patients prevents the transmission of bacteria from patient to patient. It is important for healthcare professionals to understand that gloves should not be used in place of handwashing. After removing gloves, healthcare professionals should always follow handwashing guidelines.
Gowns and masks also help prevent HAIs. Gowns worn in patient rooms and in operating rooms prevent infectious microbes from being carried from patient to patient on healthcare workers’ clothing. Masks are used to prevent microbes from being transmitted via the respiratory tract. In 2011, the Food and Drug Administration (FDA) approved a new type of N95 respirator for single-use that kills methicillin-resistant Staphlococcus aureus (MRSA), Streptococcus pyogenes, and Haemophillus influenzae. This specialty mask incorporates an antimicrobial agent into the fiber of the mask. The mask eliminates 99.99% of bacteria on its surface within 1 hour and traps and kills additional microbes in the mask’s middle filtration layers.
Transmission-Based Precautions
The topic of PPE is not complete without a review of contact, droplet, and airborne precautions. Most nurses are familiar with the types of precautions used to prevent infection transmission. Even so, nurses, as well as other healthcare professionals, can become lax in their use of precautions, increasing the incidence of HAI.
Contact precautions are designed to reduce the risk of transmission of infectious organisms by direct or indirect contact with the patient or the patient’s environment. Transmission can occur through direct skin-to-skin contact from an infected person or host or through indirect contact of a susceptible host with a contaminated intermediate object or fomite in the environment. To comply with contact precautions, healthcare professionals should follow these guidelines:
- Wear gowns and gloves for all patient contact or contact with potentially contaminated areas of the patient’s environment (e.g., bedrails, furniture, medical equipment).
- Put on PPE before entering the patient’s room and remove it before leaving the room to contain any potential microbes.
- Whenever possible, patients on contact precautions should be admitted to private rooms. Infection control personnel should be consulted before cohorting patients if a private room is not available.
- Transport patients only when necessary for diagnosis or treatment and weigh the risk of infection against the need for transport. When transporting a patient, place the patient in a clean gown and cover the patient with linen per facility policy.
Transmission of microbes by droplet involves contact of conjunctivae of the mucous membranes of the nose or mouth of a susceptible person with large particle droplets. Droplets are generated from the source patient primarily during coughing, sneezing, or talking or during certain procedures, such as suctioning and bronchoscopy. Transmission of infection by large particle droplets requires close contact between the source patient and the susceptible host. Droplets do not remain suspended in the air and generally travel only short distances–up to 3 feet. For this reason, special ventilation systems and air handling are not required for droplet precautions. To properly follow droplet precautions, healthcare professionals should follow these guidelines:
- Wear a surgical mask for close contact (within 3 feet) with patients on droplet precautions; respirators are not necessary.
- Admit the patient to a private room, if possible. Infection control personnel should be consulted before cohorting patients if a private room is not available.
- When transporting patients on droplet precautions, place a mask on them and instruct them in proper cough etiquette.
Airborne precautions are designed to prevent transmission of infectious organisms that remain infectious over long distances if they become suspended in the air. Airborne transmission occurs when there is dissemination of either airborne droplet nuclei (small particle residue of evaporated droplets) or dust particles containing the infectious agent. Airborne organisms are transmitted by air currents and may be inhaled or deposited on a susceptible host within the same room as a source patient or even a long distance away from the source patient. To prevent airborne transmission, healthcare professionals should follow proper airborne precautions:
- Admit the patient to a room with special air handling and ventilation systems. These airborne infection isolation rooms (AIIRs) use negative airflow relative to hallways or surrounding areas to prevent air from escaping the room. The rooms complete 12 air exchanges per hour in buildings with new construction and renovation or 6 air exchanges per hour in existing facilities. Air exhausted from AIIRs must be directly exhausted to the outside or put through a HEPA filtration system before returning to the building.
- Don the proper PPE when entering an isolation room, including an N95 mask–a mask approved to filter 95% of airborne particles. Healthcare professionals must not confuse N95 respirators with surgical masks. They are not the same. Surgical masks cannot filter small particles and do not prevent leakage around the edges when the user inhales. To identify an N95 mask, look for the manufacturer’s name, part number (P/N), the protection provided by the filter, and the letters NIOSH or the NIOSH logo written on either the outside front, the exhalation valve, or the straps of the mask.
- Be sure to be fit-tested for an N95 mask before you care for anyone requiring airborne precautions. Facilities that have AIIRS must have a facility-wide respiratory protection program that includes education of workers on respirator use with appropriate fit testing and user seal checks.
- Limit patient transport to medically necessary procedures only. If the patient must be transported, skin lesions must be covered and the patient must wear a surgical mask and observe respiratory hygiene/cough etiquette.
Environmental Decontamination
Some bacteria do not need a living host to survive. Microbes such as MRSA, vancomycin-resistant enterococci (VRE), and C. difficile can survive for long periods on environmental surfaces, such as bedrails and phones. After being contaminated, these environmental surfaces become the source of infection. A clean healthcare environment is essential for prevention of infection from these organisms.
Cleaning and disinfection of the patient environment includes high touch surfaces, such as bedrails, carts, toilets, and doorknobs, as well as general housekeeping surfaces, such as floors, walls, and blinds. Proper disinfection and sterilization of medical and surgical instruments and devices are also vital in the prevention of HAIs.
Agents used by facilities that are designated by the Environmental Protection Agency (EPA) as hospital-grade detergents/disinfectants must be able to inactivate specific organisms, such as staphylococci and streptococci. These agents must be chosen carefully to determine if they are effective in killing pathogens.
Nurse Staffing and Burnout and HAI
A recent study in the American Journal of Infection Control showed a correlation between nurse staffing ratios, nurse burnout, and HAIs. According to the researchers, the higher the nurse-patient ratio, the higher the likelihood of occurrence of a HAI due to increased demands on nurses and resulting burnout. The study showed that increasing a nurse’s patient load by one patient increased the incidence of urinary tract infections and SSIs. The authors of this study suggest that healthcare facilities decrease nurse burnout to decrease the incidence of HAIs.
Surveillance of HAIs
Infection surveillance helps experts identify infectious disease, spot trends in infections, and create treatment and eradication goals. The federal government provides resources to estimate and track HAIs affecting patients and healthcare personnel. The National Healthcare Safety Network (NHSN), the largest HAI reporting system in the United States collects data from participating institutions, including acute care hospitals, long-term acute care hospitals, psychiatric hospitals, rehabilitation hospitals, outpatient dialysis centers, ambulatory surgery centers, and long-term care facilities. Currently, more than 9,000 institutions participate in the NHSN to share infectious disease data in a timely manner. As the scope of HAI reporting is extended, it is the goal of the NHSN to target prevention, improve patient outcomes, and reduce healthcare costs, with the ultimate goal being elimination of all HAIs.
Another national resource, the Emerging Infections Programs (EIP), consists of a network of state health departments and their academic medical center partners. These agencies collaborate to answer questions regarding emerging HAI threats, advanced infection-tracking methods, and antibiotic resistance in the United States.
Conclusion
The medical profession has come a long way in its progress toward elimination of HAIs, but there is still a long way to go. Understanding common pathogens, knowing risk factors of HAIs, and implementing preventative measures are key ways healthcare staff can help keep patients safe. Diligence and attention to proper hand hygiene, environmental disinfection, use of proper transmission-based precautions, and the correct use of PPE will help prevent the spread of these infections. With perseverance, healthcare workers can be instrumental in reaching the ultimate goal of zero HAIs.
Resources for Part I throught V.
For more information on isolation precautions including standard and transmission-based precautions, refer to the NCCE course titled Science of Infection Control Principles available at www.nursece.com.
For general information on common HAIs, visit the CDC’s website at www.cdc.gov.
For more information on prevention strategies for VAP, refer to the publication Strategies to Prevent Ventilator-Associated Pneumonia in Acute Care Hospitals, available at http://www.jstor.org/stable/10.1086/591062.
For more information on isolation precautions, see Guidelines for Isolation Precautions: Preventing Transmission of Infectious Agents in Healthcare Settings 2007, available at www.cdc.gov.
For more information on infection control in the LTCF, see the SHEA/APIC Guideline: Infection Control in the Long-Term Care Facility, available at http://www.jstor.org/stable/10.1086/592416.
For more information on developing strategies for measurement of hand hygiene, refer to the Joint Commission’s monograph titled Measuring Hand Hygiene Adherence: Overcoming the Challenges, available at www.jointcomission.org.
For more information on the selection and proper use of disinfection and sterilization materials, see the Guideline for Disinfection and Sterilization in Healthcare Facilities, 2008, available on the CDC website at www.cdc.gov.
Top recommendations and detailed tool kits for preventing healthcare–associated infections, including urinary tract, surgical-site, Clostridium difficile, and central line–associated bloodstream infections outside the ICU are available at http://www.cdc.gov/hicpac/pdf/guidelines/bsi-guidelines-2011.pdf.
QUOTE FOR THURSDAY:
“Proper Handwashing is the single most effective way to prevent the transmission of infection. To understand the effects of handwashing, it is important to consider the environment on human skin. Microorganisms found on the skin are classified as either resident flora, colonizing flora (normal flora), or transient flora.“
CDC Centers for Disease Control and Prevention
Part IV Acquired Hospital/Healthcare Infections-Long Term Healthcare Facilities
Long-Term Care Facilities
Long-term care facilities (LTCFs), including rehabilitation centers, psychiatric hospitals, and residences for the disabled, differ from acute care hospitals in many ways. They are essentially a home for their residents, but many residents are often transferred between the LTCF and acute treatment hospitals as health needs arise. Because of their nature, LTCFs pose some unique risks for HAIs.
Nursing homes and skilled nursing facilities for the care of the elderly are the most common type of LTCF. In the United States, about 1.5 million people reside in 16,100 nursing homes. It is estimated that 765,000 to 2.8 million of these residents suffer from infections each year. Risk factors for infection are specific in this vulnerable population and include advanced age, incontinence, immobility, lower immunity, dysphagia, age-related skin changes, frequent hospitalizations, malnutrition, and underlying chronic illness.
Because of the compromised nature of these patients, they suffer from a number of HAIs. Urinary tract infections are the most common type of infection reported in LTCFs and can arise due to patient illness or incontinence. Pneumonia, influenza, and lower respiratory tract infections account for significant morbidity and mortality in residents of LTCFs. C. difficile infection is of particular importance in this population as it is the primary cause of diarrhea in nursing homes. Skin, soft tissue, and wound infections are the third most common type of infection in LTCF residents and include infected pressure ulcers and cellulitis, often with group A Streptococci and MRSA. Other infections include viral hepatitis, tuberculosis (TB), and conjunctivitis.
LTCFs face a variety of challenges associated with HAIs. Compared to acute care facilities, nursing homes frequently lack properly trained infection control personnel, and many of these staff members work only part-time on infection control regardless of the size of the facility. Few have a certification in infection control. LTCFs may also have a limited staff, a high staff turnover, problems with funding, and limited information technology (IT) resources, including a computer system that is integrated with diagnostic labs and radiology centers. Nurses in LTCFs must be particularly vigilant in protecting their patients from HAIs.
Enlisting the Help of the Patient and Family
Don’t underestimate the importance of patients and their family members and visitors. With a little education, they can help facilities decrease the spread of infection.
- Educate patients and families on the type of infection diagnosed in the patient, proper hand hygiene, and appropriate precautions, such as contact, droplet, or airborne.
- Keep materials for precautions readily available to visitors, such as gowns, gloves, and masks.
- Encourage all visitors to wash their hands properly before and after visiting with the patient.
- Post instructions on proper handwashing where visitors can see them.
- Keep adequate supplies of hand sanitizer in hallways, near doors, and by elevators and encourage visitors to use them.
Prevention Is The Key=
The Joint Commission includes infection prevention as one of its National Patient Safety Goals in hospitals, behavioral care facilities, and ambulatory care facilities, as well as home health care. Specifically, the Joint Commission emphasizes handwashing as key to infection prevention. Although hand hygiene and transmission precautions are routine in healthcare facilities, a review of evidence-based practice can remind healthcare professionals of the process of and rationale for these procedures.
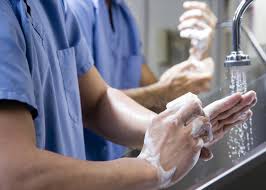
Proper Hand Hygiene
Handwashing is the single most effective way to prevent the transmission of infection. To understand the effects of handwashing, it is important to consider the environment on human skin. Microorganisms found on the skin are classified as either resident flora, colonizing flora (normal flora), or transient flora. Colonization is the presence of microorganisms in or on a host with growth and multiplication. A person who carries an organism with no clinical signs or symptoms of disease is said to be colonized with that organism. Resident organisms rarely cause infections unless they are introduced into deep tissues through invasive procedures or if the patient is severely immunocompromised. These organisms are usually aerobic, gram positive, and not easily removed by handwashing. Examples include Staphlococcus epidermidis and Corynebacterium spp.
Transient flora are organisms that are recent contaminants that survive only a short time, generally fewer than 24 hours on the skin. They readily cause infection and are most frequently associated with HAIs. These organisms are usually anaerobic, gram negative, and easily removed by handwashing. Examples include streptococci, E. coli, Candida spp., and Klebsiella pneumonia.
An overwhelming amount of literature cites inadequate handwashing in the transfer of organisms such as Staphylococcus, Enterobacteriaceae, Pseudomonas, and Klebsiella in healthcare facilities. Inadequate handwashing also places healthcare workers at risk for viral diseases, such as hepatitis and HIV, and multiple bacterial infections, such as those caused by staphylococci and streptococci.
Various organizations are at work to promote consistent and effective hand hygiene in the healthcare workplace. One agency, the World Health Organization (WHO), created the Five Moments for Hand Hygiene to simplify the timing of handwashing. This handwashing protocol dictates times for hand hygiene within the sequence of patient care to yield the maximum opportunity for patient safety. According to the WHO, the healthcare workers should wash their hands at the following “moments”:
- Before touching a patient
- Before a clean or aseptic procedure is begun
- After exposure to a body fluid
- After touching a patient
- After touching patient surroundings
The theory behind this approach is that if handwashing occurs at the precise time it is needed, transmission of microbes will be halted and patient harm will be prevented. Proponents of the WHO approach hope that the methods taught will stick with healthcare workers and handwashing compliance will increase enough to become an unconscious habit.
The CDC recommends appropriate timing and effective materials for handwashing in the following guidelines for hand hygiene during the delivery of healthcare:
- Hands should be washed with either a nonmicrobial soap and water or, in the following situations, with an antimicrobial soap and water:
- When hands are visibly dirty
- When hands are contaminated with blood or body fluids
- When hands are contaminated with protein-based substances
- When there has been contact with spore-forming bacteria, such as Clostridium difficile (The physical action of handwashing using friction and running water is more likely to remove the spores. Alcohols, chlorhexidine, and other antimicrobial agents used in antiseptic hand rubs have virtually no activity against spores. It should also be noted that alcohol-based hand sanitizers have virtually no effect on reducing the number of genomic copies of Norovirus spp. Hands should be vigorously washed with soap and water when outbreaks of infectious diarrhea or gastrointestinal illness have occurred and Norovirus is the known or suspected agent.)
- When hands are not visibly soiled, or after soil is removed with soap and water, the preferred method of handwashing, or more specifically hand decontamination, is with an alcohol-based hand rub. Hand decontamination with antimicrobial agents (hand asepsis) is indicated for removing or destroying transient organisms. Antibacterial soap and water can also be used. Hands should be decontaminated at the following times:
- Before direct contact with all patients and before donning gloves and performing invasive procedures
- After contact with blood, body fluids, excretions, mucous membranes, nonintact skin, or wound dressings
- After contact with patient intact skin (e.g., when taking blood pressure)
- During patient care, if hands are moving from a contaminated body site to a clean body site
- After contact with inanimate objects and medical equipment near the patient (e.g., bedrails, IV pumps, computer keyboards)
- After removing gloves and other personal protective equipment (PPE)
- Before preparing or eating food
- After contact with one’s own body fluids (e.g., nose blowing, sneezing, using the bathroom)
The use of antiseptic hand rub has brought into question the effectiveness of soap and water. Studies show that when hands of healthcare workers are heavily contaminated with pathogens, alcohol-based antiseptic hand rub prevents transmission of the pathogen much more effectively than plain soap and water handwashing. In a study of the transfer of gram-negative bacilli from the hands of nurses to a piece of catheter material, transfer of the bacilli occurred 2 out of 12 times (17%) when an alcohol-based hand rub was used for hand hygiene, compared to 11 out of 12 times (92%) when plain soap and water were used for hand hygiene. For standard handwashing or hand asepsis, the CDC recommends healthcare workers use alcohol-based products over plain soap or antimicrobial soap, except in the cases listed in the guidelines outlined earlier.
How you wash your hands also matters. Proper handwashing technique must be used to decrease the transmission of infection-causing organisms. Simply rinsing one’s hands with cool water and briefly wiping them dry will not reduce the rate of transmission. To reduce the number of transient organisms, hands must be washed with a sufficient amount of product, with the correct technique, and for a sufficient length of time. The following steps should be taken to ensure proper handwashing:
- When using soap and water:
- Wet hands and apply a sufficient amount of soap (3 ml).
- Rub hands vigorously to create a lather, scrubbing all surfaces of both hands, including backs of hands, wrists, between fingers, and, especially, thumbs and under fingernails. Continue for at least 20 to 30 seconds.
- Rinse hands well under running water.
- Dry hands with a paper towel, and if possible use the paper towel to turn off the faucet on sinks that do not have foot controls or automatic shut-off sensors.
- When using an alcohol-based hand rub:
- Apply the rub to the palm of one hand.
- Rub hands together, wetting all surfaces and focusing on fingernails and fingertips.
- Continue until hands are dry. Drying time should take a minimum of 15–20 seconds if a sufficient amount of rub was applied. If hands are dry in less than 15 seconds, an insufficient amount of product was used.
In addition to maintaining, monitoring, and promoting correct hand hygiene, it is also important for healthcare facilities to perform self-assessments on hand hygiene. The Joint Commission (the accrediting agency for more than 19,000 healthcare organizations) in collaboration with the CDC and other organizations outlined the rationale for self-assessment of handwashing in healthcare facilities:
- To assess the performance of staff members and educate them in real time
- To assess an institution’s level and quality of practice for regulatory or accreditation purposes
- To measure an institution’s performance within high-risk patient populations or units
- To assess the impact of a quality improvement program that increases adherence to hand hygiene guidelines
- To compare the performance of an institution to that of other healthcare facilities
- To investigate an outbreak of infection
- To conduct a research project
- To improve patient and family perception of quality of care
Facilities that assess their effectiveness at prevention can analyze their strengths and weaknesses in prevention efforts and revise their practice to reduce infection rates and thus keep patients safe.
QUOTE FOR WEDNESDAY:
“A nosocomial infection is contracted because of an infection or toxin that exists in a certain location, such as a hospital. People now use nosocomial infections interchangeably with the terms health-care associated infections (HAIs) and hospital-acquired infections. For a HAI, the infection must not be present before someone has been under medical care.”
Healthline.com
QUOTE FOR TUESDAY:
“Each year in the United States, about a half million people get sick from C. difficile, and in recent years, C. difficile infections have become more frequent, severe and difficult to treat. People who are on breathing machines (ventilators), often used in intensive care units, are at higher risk of this type of pneumonia.”
Mayo Clinic
Part II Continuation of Types of Hospital Acquired Infections (HAIs)
Ventilator Acquired Pneumonia
C-Diff
Ventilator-Associated Pneumonia
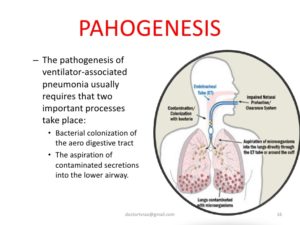
Ventilator-associated pneumonia (VAP) is an infection of the lungs that develops after a person has been on a ventilator for longer than 48 hours. The most common type of HAI contracted in the ICU, VAP occurs in as many as 28% of patients who have had mechanical ventilation. Infection occurs because the endotracheal or tracheostomy tube allows passage of microbes into the lungs. These organisms may originate from the patient’s aspirate, from the oropharynx and digestive tract, or from external sources, such as contaminated equipment and medications.
Although any microbe can be the causative agent, certain microbes are most often implicated due to increasing drug resistance. Pseudomonas aeruginosa is the most common multidrug-resistant organism responsible for VAP. Other microbes that cause VAP include Staphlococcus aureus, Klebsiella spp., Escherichia coli, Enterobacter spp., Actinobacter spp., MRSA, and Serratia marcescens. Pneumonia is considered early in onset if it occurs within the first 4 days after hospital admission. Multidrug resistant organisms are more likely to be the cause of late-onset pneumonia, defined as 5 or more days postadmission.
In addition to recent ventilation, other risk factors increase a patient’s chance of acquiring VAP, such as hospitalization or antibiotic use within the past 90 days, hospital stay greater than 5 days, hemodialysis within the past 30 days, and known circulation of multidrug-resistant organisms in the facility. Immunocompromised residents and those who reside in a nursing home or long-term care facility are also at greater risk for VAP.
Symptoms of VAP include fever, a decline in oxygenation, leukocytosis, and purulent sputum. Diagnosis of VAP is made based on comprehensive medical history, presence of infiltrates on x-ray, and positive culture of lower respiratory tract secretions (colonization of the trachea is common; thus a positive culture may not distinguish a pathogen from a colonizing organism). Symptoms of ventilator associated tracheobronchitis (VAT) with purulent secretions can mimic symptoms of VAP. VAT is a condition midway between colonization and VAP and requires antibiotic treatment.
Treatment should not be delayed while diagnostic tests are pending. Empiric treatment is vital in patients with suspected VAP and can be based on patient risk factors for multidrug-resistant organisms, known local prevalence of resistant organisms, severity of infection, and total number of days the patient was hospitalized before the onset of pneumonia. Criteria for empiric treatment include a new or progressive infiltrate on x-ray and at least two of the following conditions: fever greater than 38°C, leukocytosis or leukopenia, and purulent respiratory secretions.
If the patient has received recent doses of antibiotics, a different class of antibiotics should be used for treatment. Therapy should later be adjusted based on culture results. Unless diagnostic testing shows otherwise, initial empiric therapy should not be changed in the first 48 to 72 hours because clinical response to antibiotic therapy is not likely during this time frame. Patients should be treated with antibiotic therapy for at least 72 hours after a clinical response is attained. (For specific antibiotics and dosages, see the Infectious Disease Society of America practice guidelines for patient care at www.idsociety.org.)
To prevent VAP, the CDC recommends the following strategies:
Strategies to Prevent Aspiration
- Maintain patients in a semirecumbent position.
- Avoid gastric overdistention.
- Avoid unplanned extubation and reintubation.
- Use a cuffed endotracheal tube with inline or subglottic suctioning.
- Maintain an endotracheal cuff pressure of at least 20 cm water.
Strategies to Reduce Colonization of the Aerodigestive Tract
- When possible, use orotracheal intubation rather than nasotracheal intubation.
- Avoid histamine receptor 2 (H2)–blocking agents and proton pump inhibitors for patients who are not at high risk for developing a stress ulcer or stress gastritis.
- Perform regular oral care with an antiseptic solution.
Strategies to Minimize Contamination of Equipment
- Use sterile water to rinse reusable respiratory equipment.
- Remove condensate from ventilatory circuits, keeping the ventilatory circuit closed while you do so.
- Change the ventilatory circuit only when visibly soiled or malfunctioning.
- Store and disinfect respiratory therapy equipment properly.
In addition to these strategies, healthcare professionals should perform daily assessments of readiness to wean to minimize the duration of ventilation. Whenever possible, use noninvasive ventilation methods.
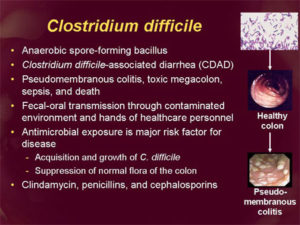
Clostridium difficile Infection
Clostridium difficile infection (CDI), or Clostridium difficile–associated disease (CDAD), is an infection of the intestines caused by the anaerobic, spore-forming, gram-positive bacillus C. difficile. (Figure 2)This microbe was first identified in 1935 when it was isolated from the stools of neonates. C. difficile produces heat-resistant spores that can remain viable on fomites in the environment for years, becoming a source of outbreaks in healthcare facilities. This bacillus also produces two types of toxins: Toxin A (an enterotoxin) and Toxin B (a cytotoxin). These toxins are responsible for the inflammatory responses of the colon, which results in loss of epithelial integrity and the production of watery diarrhea. C. difficile is the most common cause of antibiotic-associated diarrhea and pseudomembranous colitis and has proved extremely difficult to control due to new, more resistant strains.
C.difficile taken from a stool sample culture.
Source: CDC Public Health Image Library PHIL #9999, photo credit Janice Haney Carr, CDC.
- difficile is found in the intestinal tract of up to 70% of healthy infants, 1–3% of healthy adults, and in higher rates (up to 20%) in persons on antibiotic therapy. Prevalence, severity, colectomy rates, and mortality rates due to C. difficile have all risen in the past two decades, proving that this microbe has become an extremely virulent superbug that is both persistent and difficult to treat. Surveillance data from the CDC show that between 2000 and 2007 mortality from C. difficile increased by 400% due to a more virulent strain of this organism.
Studies of hospital-acquired CDI have shown that the infection is independently associated with increased risk of in-hospital death: For every 10 hospital patients who acquire a CDI, 1 patient died. Data from the CDC show that C. difficile is responsible for 337,000 infections and 14,000 deaths in the United States each year.
The greatest risk factor for CDI is the use of antibiotics, such as cephlasporins, clindamycin, or the penicillins, because these antibiotics kill the normal flora of the colon, causing overgrowth of C. difficile. Risk is increased for those taking multiple antimicrobials and those who take antimicrobials for longer time periods. Other risk factors for CDI include advanced age. Although almost half of the infections occur in persons younger than 65, most CDI-related deaths occur in the elderly. People with HIV infection, compromised immune systems, and compromised physical status are also at increased risk for CDI. Hospital admission increases one’s chance of acquiring CDI, as does gastrointestinal surgery.
Transmission of CDI occurs by the fecal-oral route.
The time between exposure to C. difficile and infection is 2 to 3 days.
Symptoms of CDI vary greatly, ranging from asymptomatic to mild (fever, malaise, and gastrointestinal symptoms, including abdominal pain and cramps, and mild to moderate foul-smelling diarrhea that is rarely bloody) to extremely severe toxic megacolon, septic shock, and even death. Complications of C. difficile include pseudomembranous colitis or fulminant colitis.
Diagnosis is based on clinical history (antibiotic use in the previous 2 months, diarrhea after 72 or more hours of hospitalization), and presence of C. difficile in the stool. Stool culture is the most sensitive test and is often used for diagnosis in the hospital setting. Colonoscopy revealing histopathology with pseudomembranous colitis is also diagnostic but not necessary in most cases.
Treatment for CDI begins with discontinuation of the antibiotic causing the infection. In many cases, this step is the only necessary treatment since normal flora can reestablish in the colon. If mild to moderate diarrhea persists, patients can be treated with either metronidazole or vancomycin. In cases of severe diarrhea, vancomycin is the drug of choice for treatment due to its history of rapid symptom resolution and overall fewer treatment failures. Although antibiotic treatment will clear the infection, it will not kill the bacterial spores. In 27% of cases, relapse occurs within 3 weeks of antibiotic termination. In extreme cases, colectomy with end ileostomy may be necessary. Treatment for asymptomatic cases is not recommended.
An innovative CDI treatment may be on the horizon. Researchers have shown that C. difficile infection arises as the result of the disruption of natural flora in the intestines, a condition known as dysbiosis. New research in the treatment of CDI involves isolating specific gut bacteria in the fecal matter of healthy individuals and incorporating it into the gut of a person with CDI to restore normal flora and cure the infection.
CDI can be catastrophic to patients and indeed to entire healthcare facilities if an outbreak occurs. To prevent CDI, follow these guidelines from the CDC:
- Immediately isolate patients with confirmed C. difficile infection and use contact precautions for the duration of diarrhea. Consider extending precautions beyond the duration of diarrhea and using presumptive contact precautions for patients with diarrhea pending confirmed C. difficile diagnosis.
- Educate healthcare personnel, patients, their families, and any visitors about C. difficile and help them maintain contact precautions.
- Follow proper handwashing techniques. Hand hygiene for C. difficile must include vigorous washing of hands with soap and water to mechanically remove spores. Alcohol-based hand rubs are not effective against C. difficile.
- Because C. difficile spores can survive on objects for long periods of time be sure to thoroughly clean and disinfect equipment and objects in the environment. Consider use of sodium hypochlorite (bleach)–containing agents or EPA-registered disinfectants with sporicidal claim for environmental cleaning.
- Enact a laboratory-based alert system for immediate notification of positive test results.
QUOTE FOR MONDAY:
“A nosocomial infection is contracted because of an infection or toxin that exists in a certain location, such as a hospital. People now use nosocomial infections interchangeably with the terms health-care associated infections (HAIs) and hospital-acquired infections.”
Healthline.com
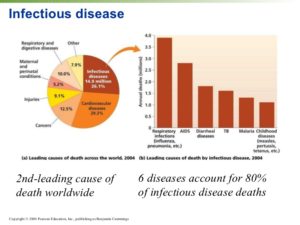
Hospital Acquired Infections-HAIs
Common Types of HAIs
Healthcare facilities, including hospitals, acute care facilities, and long-term care facilities, contain many organisms and methods of transfer of bacteria; however, certain infections occur more frequently than others in healthcare environments. In this course, the most common infections, as well as the most virulent, are discussed.
Catheter-Associated Urinary Tract Infections
Catheter-associated urinary tract infections, or CAUTIs, are the most common type of HAI, representing more than 30% of all hospital-reported infections. CAUTIs are the leading cause of secondary, hospital-acquired bloodstream infections (about 17% of hospital-acquired bacteremias), and the mortality associated with these infections is about 10%.
The most common pathogens causing CAUTIs are E. coli, Candida spp., Enterococcus spp., Pseudomonas aeruginosa, Klebsiella pneumoniae, and Enterobacter spp. Pathogens may gain access to the urinary system during insertion, manipulation, maintenance, or removal of a urinary catheter. Pathogens enter the urinary tract via the extraluminal route, by moving along the outside of the catheter in the periurethral mucous sheath, or the intraluminal route, by moving along the internal lumen of the catheter from a contained collection bag or catheter drainage tube junction.
Studies show that by the 30th day of catheterization, which is also considered the demarcation between short- and long-term catheterization, the daily risk of bacteriuria approaches 100%. Over time, a thin layer of microorganisms along with their DNA, proteins, and polysaccharides (known as extracellular polymorphic substances or EPS) form a biofilm on the surface of the catheter (see Figure 1). The longer the catheter remains in place, the greater the chance of biofilm production and thus urinary tract infection. Microbes composing a biofilm are tightly bound to the surface of the catheter and extremely resistant to antimicrobial therapy, making removal of the catheter necessary to effectively eliminate the infection.
Figure 1
Electron micrograph showing biofilm formation by Staphlococcus aureus bacteria on the inside lumen of an indwelling catheter.
Source: CDC Public Health Image Library PHIL #7483, photo credit Janice Haney Carr, CDC.
Although anyone with a urinary catheter can suffer a urinary tract infection, certain people are more at risk, including women, older adults and those with prolonged catheterization. Medical conditions that increase the risk of a CAUTI include diabetes, diarrhea, renal insufficiency, and a compromised immune system. Colonization of the catheter drainage bag can also increase a patient’s risk for a CAUTI.
Symptoms of CAUTI are often nonspecific. Patients may have fever and leukocytosis. To diagnose a CAUTI after a catheter has been removed, urine cultures are obtained from a clean-catch midstream specimen. When a catheter has been in place longer than 2 weeks, the catheter should be replaced first, and then the urine specimen should be obtained from the new catheter. In some cases, removal of the catheter may be the only necessary treatment. If asymptomatic bacteriuria continues after the catheter has been removed for 48 hours, antibiotic treatment may be necessary. Duration of treatment is generally 7–14 days.
To help prevent CAUTIs, the CDC recommends healthcare workers follow these guidelines:
- Insert catheters only for the appropriate indications and minimize their use in those at high risk of CAUTIs, especially the elderly, women, and immunocompromised patients.
- Leave catheters in place only for as long as needed. Remove catheters on postoperative patients as soon as possible, preferably within 24 hours unless there are appropriate indications for continued use.
- Avoid use of urinary catheters in patients and nursing home residents for the management of incontinence.
- Ensure that only properly trained persons insert and maintain catheters.
- Insert catheters using aseptic technique and sterile equipment. Use proper CDC hand hygiene and standard or appropriate isolation precautions (see discussion of these topics later in this course) when inserting or handling catheters. Perform hand hygiene immediately before and after insertion or any manipulation of the catheter site or device.
- Maintain a closed drainage system with unobstructed urine flow. Urinary catheter systems with preconnected, sealed catheter-tubing junctions are suggested for use.
- Do not clamp indwelling catheters prior to removal and do not change indwelling catheters or drainage bags at routine intervals. Catheters and drainage bags should be changed based on clinical indications, such as infection, obstruction, or when the closed system is compromised.
- Avoid use of systemic antimicrobials for routine prophylaxis of CAUTIs unless clinical indications exist. Routine screening of catheterized patients for asymptomatic bacteriuria is not recommended.
- Unless obstruction of the catheter is suspected, do not irrigate the bladder.
- Do not clean the periurethral area with antiseptics to prevent a CAUTI while the catheter is in place or instill antiseptic or antimicrobial solutions into the drainage bag. Routine hygiene (e.g., cleansing of the exposed tubing during daily bathing) is appropriate.
In addition, facility-wide quality improvement programs should be implemented to ensure appropriate use of indwelling catheters and reduce the risk of CAUTI. Nurses can assist in risk-reduction and surveillance measures by providing regular feedback of unit-specific CAUTI rates and considering alternatives to indwelling urinary catheterization.
Central Line–Associated Bloodstream Infections
Perhaps the most deadly HAI, central line–associated bloodstream infections (CLABSIs) are transmitted via a central venous catheter (CVC) directly to a patient’s bloodstream. A CVC is any intravascular catheter that terminates at or close to the heart or in one of the great vessels and is used for infusion of medications, nutrition, and blood; withdrawal of blood; hemodialysis access; and hemodynamic monitoring. CVCs may be placed in the large veins of the chest (through axillary or subclavian veins), the neck (through the internal jugular vein), or the groin (through the femoral vein).
The CDC defines a CLABSI as recovery of a pathogen from a blood culture (a single blood culture for organisms not commonly present on the skin and two or more blood cultures for organisms commonly present on the skin) in a patient who had a central line at the time of infection or within the 48-hour period before infection. The infection cannot be related to any other infection the patient might have and must not have been present or incubating when the patient was admitted to the healthcare facility.
Tens of thousands of CLABSIs occur in U.S. hospitals each year, with deadly results: Up to 25% of those diagnosed with a CLABSI will die from the infection. The cost of a CLABSI is also profound, with an estimated medical cost of more than $16,000. Fortunately, in the United States the number of ICU patients diagnosed with CLABSIs fell from 43,000 in 2001 to 18,000 in 2009. However, a significant number of these infections continue to occur in inpatient settings and in hemodialysis facilities, even though most CLABSIs, especially those occurring in ICUs, are preventable.
The most common pathogens causing CLABSIs are Staphylococcus aureus, coagulase-negative staphlococci, enterococci, Candida spp., and gram-negative bacilli. Although antimicrobial resistance remains a problem for all common pathogens, it is believed that the incidence of CLABSIs caused by methicillin-resistant Staphylococcus aureus (MRSA) has decreased in recent years because of prevention efforts. Unfortunately, drug resistance for Klebsiella pneumonia, E. coli, Pseudomonas aeruginosa, and Candida spp. is on the rise.
Certain conditions increase the risk of CLABSI, such as the density of skin flora at the site of catheter insertion. In adults, catheters inserted into a femoral vein have high colonization rates and higher rates of CLABSIs when compared to catheters inserted into internal jugular and subclavian veins. (Studies in pediatric patients have shown that catheters in femoral veins have an equivalent infection rate to that of nonfemoral catheters.) The length of time the catheter is in place also affects infection rates. The longer the patient has a central line, the more likely he or she is to acquire a bloodstream infection via the line. The type of material the catheter is made from also affects infection transmission. The rate of infection for polytetrafluoroethylene (Teflon®) or polyurethane catheters is lower than that of catheters made from polyvinylchloride or polyethylene. Infection risk is also increased in patients with concurrent infection and those treated in the ICU.
Symptoms of a CLABSI may include fever, chills, hypotension, and pain or erythema at the catheter site. Diagnosis is made based on signs and symptoms in conjunction with lab confirmation of a recognized pathogen cultured from one or more blood cultures, with the cultured pathogen not being related to an infection at another site. Treatment of CLABSI includes a multilevel approach: administration of an empiric antibiotic, such as vancomycin; isolation of the causative organism with narrowing of the antibiotic choice; and determination of whether to remove the infected catheter. Patients in an immunocompromised state and those with severe illness, sepsis, a femoral catheter, or an infection with a suspected multidrug-resistant organism may require additional empiric antibiotics until lab results are available.
Prevention is key to eliminating CLABSIs in healthcare facilities. The CDC recommends healthcare professionals follow these guidelines to reduce CLABSIs in the workplace:
- Choose proper central line insertion sites to minimize infections and mechanical complications. Avoid the femoral site in adult patients.
- Follow proper insertion practices, including complying with hand hygiene recommendations; using maximum sterile barrier precautions, including mask, cap, gown, sterile gloves, and a sterile full-body drape; performing adequate skin antisepsis with > 0.5% chlorhexidine with alcohol; and covering the site with sterile gauze or sterile, transparent, semipermeable dressings.
- When accessing the line, scrub the hub/port with an appropriate antiseptic (e.g., chlorhexidine, povidone iodine, an iodophor, or 70% alcohol) and access lines only with sterile devices.
- Replace dressings that are wet, soiled, or dislodged. When changing dressings, use aseptic technique, including clean and sterile gloves, as per facility policy.
- Perform daily audits to determine if a central line is still needed, and remove unnecessary central lines.
In addition healthcare facilities should also bundle CVC supplies into “central line kits” to ensure items are readily available for use and thus maintain sterility and aseptic technique. Facilities should also consider implementing chlorhexidine bathing of ICU patients and use of antimicrobial-impregnated catheters and chlorhexidine-impregnated dressings.
Surgical Site Infections
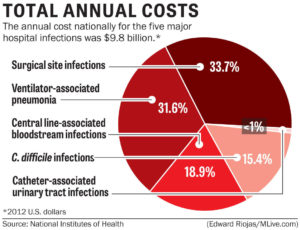
Anytime the skin barrier is broken, the risk for infection rises, so it is only logical that surgery will increase a patient’s risk for a HAI. Surgical site infections (SSIs) occur at the location where a surgery was previously performed. To be diagnosed as a SSI, the infection must occur within 30 days of surgery if no implant is left in place or within 1 year if the implant is in place and the infection appears to be related to the operative procedure. SSIs occur in 2–5% of surgeries and number about 300,000 per year. The CDC estimates costs for SSIs to be $3,000–$29,000 per infection, with 7–10 additional days of hospital stay per patient, and an overall cost to the healthcare industry of $10 billion annually.
SSIs vary in severity. They may be superficial incisional infections, which involve only the skin and subcutaneous tissue at the incision site, or they may be deep incisional infections, which occur in the deep soft tissues of the muscle or fascia. An organ or space SSI occurs in any part of the body (excluding skin, muscle, and fascia) that is opened or manipulated during the operative procedure.
Pathogens responsible for SSIs may originate from the patient’s skin, mucous membranes, or gastrointestinal tract, or they may be transmitted via hospital personnel, the hospital environment, or medical devices and surgical tools. Common causative organisms include Staphylococcus aureus, coagulase negative staphylococci, Enterococcus spp., Escherichia coli, Pseudomonas aeruginosa, and Enterobacter spp.
Many factors increase the risk of SSIs. Long surgeries (duration greater than 2 hours), emergency surgery, and abdominal surgery carry a higher infection risk as does preoperative shaving of the surgical site. Older adults, obese people, and those with diabetes, a concurrent disease, or a compromised immune system are at greater risk for SSIs. Colonization of the nares with Staphylococcus aureus creates an increased risk for SSI, as do radiation, chemotherapy, steroid use, and smoking.
Symptoms of a SSI may include erythema, pain, tenderness, edema, heat, and abscess formation or purulent discharge at the surgery site. The patient may also be febrile. Treatment may include drainage of abscesses, surgical debridement, decolonization strategies, and appropriate antibiotic therapy.
To prevent SSIs, the CDC issued the following recommendations for healthcare professionals:
Prior to Surgery
- Administer prophylactic antibiotics in accordance with evidence-based standards and guidelines. Appropriate agents should be selected on the basis of surgical procedure, the most common SSI pathogens for the procedure, and published recommendations. These medications should be administered within 1 hour prior to incision; vancomycin and fluoroquinolones should be given 2 hours prior.
- Whenever possible, identify and treat remote infections before elective surgery, or postpone surgery until the infection has resolved.
- Prep skin using an appropriate antiseptic agent and proper technique. Do not remove hair at the operative site unless it will interfere with the operation. If hair must be removed, razors should not be used. Clip hair or use a depilatory agent.
- For colorectal surgery patients, mechanically prepare the colon (enemas, cathartic agents) and administer nonabsorbable antimicrobial agents given in divided doses on the day prior to surgery.
- If your patient is undergoing elective cardiac and other procedures (i.e., orthopedic, neurosurgery procedures with implants), perform a nasal screen and decolonize only S. aureus carriers with preoperative mupirocin therapy.
- Screen preoperative blood glucose levels and maintain tight glucose control postoperative day 1 and day 2 in patients undergoing elective procedures (e.g., bypass surgeries, arthroplasties, spinal fusions).
During Surgery
- Keep operating room doors closed during surgery except as needed for passage of equipment, personnel, and the patient.
- Consider redosing antibiotics at the 3-hour interval in procedures lasting longer than 3 hours and adjust the antimicrobial prophylaxis dose for patients with a body mass index greater than 30.
- Consider using at least 50% fraction of inspired oxygen intraoperatively and immediately postoperatively in select procedures.
After Surgery
- Protect the primary closure incision with a sterile dressing for 24–48 hours postop.
- Maintain immediate postoperative normothermia.
- For cardiac surgeries, control blood glucose levels during the immediate postoperative period. Glucose level should be measured at 6:00 a.m. on postop day 1 and day 2 (procedure day is postop day 0). Postop glucose level should be maintained at < 200mg/dL.
- Discontinue antibiotics according to evidence-based standards and guidelines (within 24 hours after surgery end time, or 48 hours for cardiac surgeries).
(Continuation of Part II tomorrow).
QUOTE FOR THE WEEKEND:
“Stress costs American industry more than $300 billion annually.
The lifetime prevalence of an emotional disorder is more than 50%, often due to chronic, untreated stress reactions.”
CDC