Common Types of HAIs
Healthcare facilities, including hospitals, acute care facilities, and long-term care facilities, contain many organisms and methods of transfer of bacteria; however, certain infections occur more frequently than others in healthcare environments. In this course, the most common infections, as well as the most virulent, are discussed.
Catheter-Associated Urinary Tract Infections
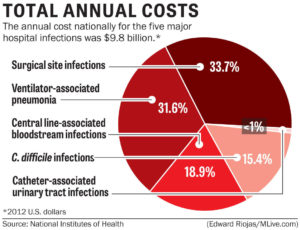
Catheter-associated urinary tract infections, or CAUTIs, are the most common type of HAI, representing more than 30% of all hospital-reported infections. CAUTIs are the leading cause of secondary, hospital-acquired bloodstream infections (about 17% of hospital-acquired bacteremias), and the mortality associated with these infections is about 10%.
The most common pathogens causing CAUTIs are E. coli, Candida spp., Enterococcus spp., Pseudomonas aeruginosa, Klebsiella pneumoniae, and Enterobacter spp. Pathogens may gain access to the urinary system during insertion, manipulation, maintenance, or removal of a urinary catheter. Pathogens enter the urinary tract via the extraluminal route, by moving along the outside of the catheter in the periurethral mucous sheath, or the intraluminal route, by moving along the internal lumen of the catheter from a contained collection bag or catheter drainage tube junction.
Studies show that by the 30th day of catheterization, which is also considered the demarcation between short- and long-term catheterization, the daily risk of bacteriuria approaches 100%. Over time, a thin layer of microorganisms along with their DNA, proteins, and polysaccharides (known as extracellular polymorphic substances or EPS) form a biofilm on the surface of the catheter (see Figure 1). The longer the catheter remains in place, the greater the chance of biofilm production and thus urinary tract infection. Microbes composing a biofilm are tightly bound to the surface of the catheter and extremely resistant to antimicrobial therapy, making removal of the catheter necessary to effectively eliminate the infection.
Figure 1
Electron micrograph showing biofilm formation by Staphlococcus aureus bacteria on the inside lumen of an indwelling catheter.
Source: CDC Public Health Image Library PHIL #7483, photo credit Janice Haney Carr, CDC.
Although anyone with a urinary catheter can suffer a urinary tract infection, certain people are more at risk, including women, older adults and those with prolonged catheterization. Medical conditions that increase the risk of a CAUTI include diabetes, diarrhea, renal insufficiency, and a compromised immune system. Colonization of the catheter drainage bag can also increase a patient’s risk for a CAUTI.
Symptoms of CAUTI are often nonspecific. Patients may have fever and leukocytosis. To diagnose a CAUTI after a catheter has been removed, urine cultures are obtained from a clean-catch midstream specimen. When a catheter has been in place longer than 2 weeks, the catheter should be replaced first, and then the urine specimen should be obtained from the new catheter. In some cases, removal of the catheter may be the only necessary treatment. If asymptomatic bacteriuria continues after the catheter has been removed for 48 hours, antibiotic treatment may be necessary. Duration of treatment is generally 7–14 days.
To help prevent CAUTIs, the CDC recommends healthcare workers follow these guidelines:
- Insert catheters only for the appropriate indications and minimize their use in those at high risk of CAUTIs, especially the elderly, women, and immunocompromised patients.
- Leave catheters in place only for as long as needed. Remove catheters on postoperative patients as soon as possible, preferably within 24 hours unless there are appropriate indications for continued use.
- Avoid use of urinary catheters in patients and nursing home residents for the management of incontinence.
- Ensure that only properly trained persons insert and maintain catheters.
- Insert catheters using aseptic technique and sterile equipment. Use proper CDC hand hygiene and standard or appropriate isolation precautions (see discussion of these topics later in this course) when inserting or handling catheters. Perform hand hygiene immediately before and after insertion or any manipulation of the catheter site or device.
- Maintain a closed drainage system with unobstructed urine flow. Urinary catheter systems with preconnected, sealed catheter-tubing junctions are suggested for use.
- Do not clamp indwelling catheters prior to removal and do not change indwelling catheters or drainage bags at routine intervals. Catheters and drainage bags should be changed based on clinical indications, such as infection, obstruction, or when the closed system is compromised.
- Avoid use of systemic antimicrobials for routine prophylaxis of CAUTIs unless clinical indications exist. Routine screening of catheterized patients for asymptomatic bacteriuria is not recommended.
- Unless obstruction of the catheter is suspected, do not irrigate the bladder.
- Do not clean the periurethral area with antiseptics to prevent a CAUTI while the catheter is in place or instill antiseptic or antimicrobial solutions into the drainage bag. Routine hygiene (e.g., cleansing of the exposed tubing during daily bathing) is appropriate.
In addition, facility-wide quality improvement programs should be implemented to ensure appropriate use of indwelling catheters and reduce the risk of CAUTI. Nurses can assist in risk-reduction and surveillance measures by providing regular feedback of unit-specific CAUTI rates and considering alternatives to indwelling urinary catheterization.
Central Line–Associated Bloodstream Infections
Perhaps the most deadly HAI, central line–associated bloodstream infections (CLABSIs) are transmitted via a central venous catheter (CVC) directly to a patient’s bloodstream. A CVC is any intravascular catheter that terminates at or close to the heart or in one of the great vessels and is used for infusion of medications, nutrition, and blood; withdrawal of blood; hemodialysis access; and hemodynamic monitoring. CVCs may be placed in the large veins of the chest (through axillary or subclavian veins), the neck (through the internal jugular vein), or the groin (through the femoral vein).
The CDC defines a CLABSI as recovery of a pathogen from a blood culture (a single blood culture for organisms not commonly present on the skin and two or more blood cultures for organisms commonly present on the skin) in a patient who had a central line at the time of infection or within the 48-hour period before infection. The infection cannot be related to any other infection the patient might have and must not have been present or incubating when the patient was admitted to the healthcare facility.
Tens of thousands of CLABSIs occur in U.S. hospitals each year, with deadly results: Up to 25% of those diagnosed with a CLABSI will die from the infection. The cost of a CLABSI is also profound, with an estimated medical cost of more than $16,000. Fortunately, in the United States the number of ICU patients diagnosed with CLABSIs fell from 43,000 in 2001 to 18,000 in 2009. However, a significant number of these infections continue to occur in inpatient settings and in hemodialysis facilities, even though most CLABSIs, especially those occurring in ICUs, are preventable.
The most common pathogens causing CLABSIs are Staphylococcus aureus, coagulase-negative staphlococci, enterococci, Candida spp., and gram-negative bacilli. Although antimicrobial resistance remains a problem for all common pathogens, it is believed that the incidence of CLABSIs caused by methicillin-resistant Staphylococcus aureus (MRSA) has decreased in recent years because of prevention efforts. Unfortunately, drug resistance for Klebsiella pneumonia, E. coli, Pseudomonas aeruginosa, and Candida spp. is on the rise.
Certain conditions increase the risk of CLABSI, such as the density of skin flora at the site of catheter insertion. In adults, catheters inserted into a femoral vein have high colonization rates and higher rates of CLABSIs when compared to catheters inserted into internal jugular and subclavian veins. (Studies in pediatric patients have shown that catheters in femoral veins have an equivalent infection rate to that of nonfemoral catheters.) The length of time the catheter is in place also affects infection rates. The longer the patient has a central line, the more likely he or she is to acquire a bloodstream infection via the line. The type of material the catheter is made from also affects infection transmission. The rate of infection for polytetrafluoroethylene (Teflon®) or polyurethane catheters is lower than that of catheters made from polyvinylchloride or polyethylene. Infection risk is also increased in patients with concurrent infection and those treated in the ICU.
Symptoms of a CLABSI may include fever, chills, hypotension, and pain or erythema at the catheter site. Diagnosis is made based on signs and symptoms in conjunction with lab confirmation of a recognized pathogen cultured from one or more blood cultures, with the cultured pathogen not being related to an infection at another site. Treatment of CLABSI includes a multilevel approach: administration of an empiric antibiotic, such as vancomycin; isolation of the causative organism with narrowing of the antibiotic choice; and determination of whether to remove the infected catheter. Patients in an immunocompromised state and those with severe illness, sepsis, a femoral catheter, or an infection with a suspected multidrug-resistant organism may require additional empiric antibiotics until lab results are available.
Prevention is key to eliminating CLABSIs in healthcare facilities. The CDC recommends healthcare professionals follow these guidelines to reduce CLABSIs in the workplace:
- Choose proper central line insertion sites to minimize infections and mechanical complications. Avoid the femoral site in adult patients.
- Follow proper insertion practices, including complying with hand hygiene recommendations; using maximum sterile barrier precautions, including mask, cap, gown, sterile gloves, and a sterile full-body drape; performing adequate skin antisepsis with > 0.5% chlorhexidine with alcohol; and covering the site with sterile gauze or sterile, transparent, semipermeable dressings.
- When accessing the line, scrub the hub/port with an appropriate antiseptic (e.g., chlorhexidine, povidone iodine, an iodophor, or 70% alcohol) and access lines only with sterile devices.
- Replace dressings that are wet, soiled, or dislodged. When changing dressings, use aseptic technique, including clean and sterile gloves, as per facility policy.
- Perform daily audits to determine if a central line is still needed, and remove unnecessary central lines.
In addition healthcare facilities should also bundle CVC supplies into “central line kits” to ensure items are readily available for use and thus maintain sterility and aseptic technique. Facilities should also consider implementing chlorhexidine bathing of ICU patients and use of antimicrobial-impregnated catheters and chlorhexidine-impregnated dressings.
Surgical Site Infections
Anytime the skin barrier is broken, the risk for infection rises, so it is only logical that surgery will increase a patient’s risk for a HAI. Surgical site infections (SSIs) occur at the location where a surgery was previously performed. To be diagnosed as a SSI, the infection must occur within 30 days of surgery if no implant is left in place or within 1 year if the implant is in place and the infection appears to be related to the operative procedure. SSIs occur in 2–5% of surgeries and number about 300,000 per year. The CDC estimates costs for SSIs to be $3,000–$29,000 per infection, with 7–10 additional days of hospital stay per patient, and an overall cost to the healthcare industry of $10 billion annually.
SSIs vary in severity. They may be superficial incisional infections, which involve only the skin and subcutaneous tissue at the incision site, or they may be deep incisional infections, which occur in the deep soft tissues of the muscle or fascia. An organ or space SSI occurs in any part of the body (excluding skin, muscle, and fascia) that is opened or manipulated during the operative procedure.
Pathogens responsible for SSIs may originate from the patient’s skin, mucous membranes, or gastrointestinal tract, or they may be transmitted via hospital personnel, the hospital environment, or medical devices and surgical tools. Common causative organisms include Staphylococcus aureus, coagulase negative staphylococci, Enterococcus spp., Escherichia coli, Pseudomonas aeruginosa, and Enterobacter spp.
Many factors increase the risk of SSIs. Long surgeries (duration greater than 2 hours), emergency surgery, and abdominal surgery carry a higher infection risk as does preoperative shaving of the surgical site. Older adults, obese people, and those with diabetes, a concurrent disease, or a compromised immune system are at greater risk for SSIs. Colonization of the nares with Staphylococcus aureus creates an increased risk for SSI, as do radiation, chemotherapy, steroid use, and smoking.
Symptoms of a SSI may include erythema, pain, tenderness, edema, heat, and abscess formation or purulent discharge at the surgery site. The patient may also be febrile. Treatment may include drainage of abscesses, surgical debridement, decolonization strategies, and appropriate antibiotic therapy.
To prevent SSIs, the CDC issued the following recommendations for healthcare professionals:
Prior to Surgery
- Administer prophylactic antibiotics in accordance with evidence-based standards and guidelines. Appropriate agents should be selected on the basis of surgical procedure, the most common SSI pathogens for the procedure, and published recommendations. These medications should be administered within 1 hour prior to incision; vancomycin and fluoroquinolones should be given 2 hours prior.
- Whenever possible, identify and treat remote infections before elective surgery, or postpone surgery until the infection has resolved.
- Prep skin using an appropriate antiseptic agent and proper technique. Do not remove hair at the operative site unless it will interfere with the operation. If hair must be removed, razors should not be used. Clip hair or use a depilatory agent.
- For colorectal surgery patients, mechanically prepare the colon (enemas, cathartic agents) and administer nonabsorbable antimicrobial agents given in divided doses on the day prior to surgery.
- If your patient is undergoing elective cardiac and other procedures (i.e., orthopedic, neurosurgery procedures with implants), perform a nasal screen and decolonize only S. aureus carriers with preoperative mupirocin therapy.
- Screen preoperative blood glucose levels and maintain tight glucose control postoperative day 1 and day 2 in patients undergoing elective procedures (e.g., bypass surgeries, arthroplasties, spinal fusions).
During Surgery
- Keep operating room doors closed during surgery except as needed for passage of equipment, personnel, and the patient.
- Consider redosing antibiotics at the 3-hour interval in procedures lasting longer than 3 hours and adjust the antimicrobial prophylaxis dose for patients with a body mass index greater than 30.
- Consider using at least 50% fraction of inspired oxygen intraoperatively and immediately postoperatively in select procedures.
After Surgery
- Protect the primary closure incision with a sterile dressing for 24–48 hours postop.
- Maintain immediate postoperative normothermia.
- For cardiac surgeries, control blood glucose levels during the immediate postoperative period. Glucose level should be measured at 6:00 a.m. on postop day 1 and day 2 (procedure day is postop day 0). Postop glucose level should be maintained at < 200mg/dL.
- Discontinue antibiotics according to evidence-based standards and guidelines (within 24 hours after surgery end time, or 48 hours for cardiac surgeries).
(Continuation of Part II tomorrow).