Treatment Options for Bleeding Disorders
There are many different types of therapies for bleeding disorders, and new ones are in development. Each person may respond to a treatment in their own way, so it is important to work closely with your hematologist to find a treatment that works for you.
Gene therapy is a way of treating a genetic disease or disorder by providing people with working copies of the gene to correct the disease or disorder. There are different approaches to gene therapy, including gene transfer and gene editing.
Currently, gene therapies for Hemophilia A and Hemophilia B work differently in the body and have different results. It is important that you work with your Hemophilia Treatment Center to learn more about gene therapy, to determine if you are eligible, to make certain you understand the risks and benefits, and to ensure you have the information you need to make the best decision for you.
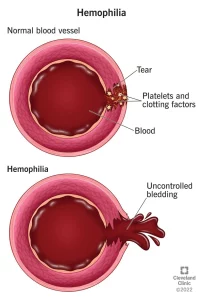
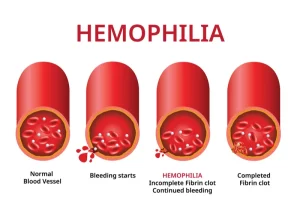
Factor replacement therapies: Often referred to as “factor,” these products use a molecule that is either similar to natural factor found in humans (recombinant) or use an actual human molecule (plasma derived.) These treatments increase the amount of factor in the body to levels that lead to better clotting, and therefore less bleeding. The therapy is taken intravenously via an injection into a vein. This process is also called “infusion.” There are two types of factor replacement therapies: standard half-life (SHL) and extended half-life (EHL)
- Standard half-life therapies: Standard half-life therapies are used to treat hemophilia A and B, some types of von Willebrand disease, and some rare factor disorders. Dosing can be anywhere from three times a week to every day, depending on the person.
- Extended half-life (EHL) therapies: EHL contains a molecule that has been modified in some way to delay the breaking down of factor in the body. This results in higher levels of factor in the body lasting for longer, resulting in less frequent infusions. How long the factor is effective in the body depends on the person. Extended half-life therapies are mostly used to treat hemophilia A and B.
- Bypassing agents are used to treat bleeds in people with hemophilia with inhibitors. These treatments contain other factors that can stimulate the formation of a clot and stop bleeding.
Non-factor replacement therapies: These products help prevent bleeding or assist in better clotting using other methods in the body besides factor replacement therapy. Non-factor replacement therapies include:
Hemophilia B gene therapy has been approved by the FDA for the treatment of adults with hemophilia B who currently use factor IX (FIX) prophylaxis therapy, or have current or historical life-threatening hemorrhage, or have repeated, serious spontaneous bleeding episodes.
Also used is the following:
- Emicizumab (Hemlibra) is a therapy used to treat hemophilia A, to prevent bleeding episodes in people both with and without inhibitors. It is known as a factor VIII(8) mimetic because it mimics, or imitates, the way factor VIII(8) works. It brings together factor IX(9) and factor X (10), which allows the blood to clot. Unlike factor replacement therapy, in which the missing factor is injected directly into a person’s vein (called an infusion), emicizumab is given by an injection under the skin, called a subcutaneous injection. Emicizumab was approved by the FDA to treat people with hemophilia A with inhibitors in 2017 and for people with hemophilia A without inhibitors in 2018.
- Desmopressin (DDAVP) is the synthetic version of vasopressin, a natural antidiuretic hormone that helps stop bleeding. In patients with mild hemophilia, it can be used for joint and muscle bleeds, for nose and mouth bleeds, and before and after surgery. It comes in an injectable form and a nasal spray. The manufacturer of DDAVP nasal spray issued a recall of all US products and does not expect to begin resupplying until 2022. DDAVP is used to treat von Willebrand disease and mild hemophilia A.
- Aminocaproic acid (Amicar) prevents the breakdown of blood clots. It is often recommended before dental procedures, and to treat nose and mouth bleeds. It is taken orally, as a tablet or liquid. MASAC recommends that a dose of clotting factor be taken first to form a clot, then aminocaproic acid, to preserve the clot and keep it from being broken down prematurely. This can be used to manage bleeding in people with hemophilia A, B and VWD.
- Genetic testing is also available for the factor VIII gene and the factor IX gene. Genetic testing of the FVIII gene finds a disease-causing mutation in up to 98 percent of individuals who have hemophilia A. Genetic testing of the FIX gene finds disease-causing mutations in more than 99 percent of individuals who have hemophilia B.
- Researchers have been working to develop a gene replacement treatment (gene therapy) for Hemophilia A. Research of gene therapy for hemophilia A is now taking place. The results are encouraging. Researchers continue to evaluate the long-term safety of gene therapies. The hope is that there will be a genetic cure for hemophilia in the future.
- HEMGENIX, came about 2023,it is a adeno-associated virus vector-based gene therapy indicated for treatment of adults with Hemophilia B (congenital Factor IX deficiency) who:
- Currently use Factor IX prophylaxis therapy, or
- Have current or historical life-threatening hemorrhage, or
- Have repeated, serious spontaneous bleeding episodes.