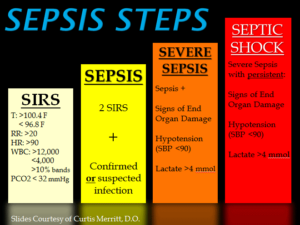
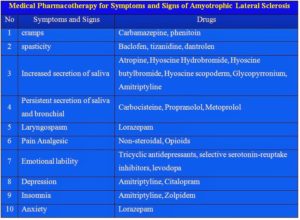
SIRS was first described by Dr. William R. Nelson, of the University of Toronto, in a presentation to the Nordic Micro Circulation meeting in Geilo, Norway-February 1983. In 1992, the American College of Chest Physicians (ACCP) and the Society of Critical Care Medicine (SCCM) introduced definitions for systemic inflammatory response syndrome (SIRS), sepsis, severe sepsis, septic shock, and multiple organ dysfunction syndrome (MODS), they are interrelated with each other in SIRS. The idea behind defining SIRS was to define a clinical response to a nonspecific insult of either infectious or noninfectious origin. SIRS is defined as 2 or more of the following variables:
-
Fever of more than 38°C (100.4°F) or less than 36°C (96.8°F)
-
Heart rate of more than 90 beats per minute
-
Respiratory rate of more than 20 breaths per minute or arterial carbon dioxide tension (PaCO 2) of less than 32 mm Hg, which is normally in our body at 35-45 mm Hg whereas the oxygen= PaO2 in our body greater than 80mm Hg for the norm.
-
Abnormal white blood cell count (>12,000/µL or < 4,000/µL or >10% immature [band] forms)
SIRS is nonspecific and can be caused by ischemia, inflammation, trauma, infection, or several insults combined. Thus, SIRS is not always related to infection but commonly is. SIRS is an inflammatory state affecting the whole body, frequently a response of the immune system to infection, but not always. It is frequently related to sepsis, a condition in which individuals meet criteria for SIRS and have a known infection.
It is the body’s response to an infectious or noninfectious insult to it. Although the definition of SIRS refers to it as an “inflammatory” response, it actually has pro- and anti-inflammatory components. SIRS describes the host response to a critical illness of infectious or noninfectious cause, such as burns, trauma, and pancreatitis. More specific definitions are as follows: Sepsis is SIRS resulting from a presumed or known site of infection. Severe sepsis is sepsis with an acute associated multiple organ failure.
What causes sepsis?
Bacterial infections are the most common cause of sepsis. Sepsis can also be caused by fungal, parasitic, or viral infections. The source of the infection can be any of a number of places throughout the body. Common sites and types of infection that can lead to sepsis include:
- The abdomen—An inflammation of the appendix (appendicitis), bowel problems, infection of the abdominal cavity (peritonitis), and gallbladder or liver infections
- The central nervous system—Inflammation or infections of the brain or the spinal cord
- The lungs—Infections such as pneumonia
- The skin—Bacteria can enter skin through wounds or skin inflammations, or through the openings made with intravenous (IV) catheters (tubes inserted into the body to administer or drain fluids). Conditions such as cellulitis (inflammation of the skin’s connective tissue) can cause sepsis.
- The urinary tract (kidneys or bladder)—Urinary tract infections are especially likely if the patient has a urinary catheter to drain urine
Who is at risk for sepsis?
Sepsis can strike anyone, but those at particular risk include:
- People with weakened immune systems
- Patients who are in the hospital
- People with pre-existing infections or medical conditions
- People with severe injuries, such as large burns or bullet wounds
- People with a genetic tendency for sepsis
- The very old or very young
What are the symptoms of sepsis?
Because of the many sites on the body from which sepsis can originate, there is a wide variety of symptoms. The most prominent are:
- Decreased urine output
- Fast heart rate
- Fever
- Or the opposite Hypothermia (very low body temperature)
- Shaking
- Chills
- Warm skin or a skin rash
- Confusion or delirium
- Hyperventilation (rapid breathing)
How is sepsis diagnosed?
A person may have sepsis if he or she has:
- A high or low white blood cell count
- A low platelet count
- Acidosis (too much acid in the blood); in the hospital what is checked is lactic acid blood level.
- A blood culture that is positive for bacteria
- Abnormal kidney or liver function
How is sepsis treated?
The most important intervention in sepsis is quick diagnosis and prompt treatment. Patients diagnosed with severe sepsis are usually placed in the intensive care unit (ICU) of the hospital for special treatment. The doctor will first try to identify the source and the type of infection, and then administer antibiotics to treat the infection. (Note: antibiotics are ineffective against infections caused by viruses; if anything what is used is antiviral medications.)
The doctor also administers IV fluids to prevent blood pressure from dropping too low. In some cases, vasopressor medications (which constrict blood vessels) are needed to achieve an adequate blood pressure. Some patients are given new drug therapies, such as activated protein C (APC). And finally, if organ failures occur, appropriate supportive care is provided (for example, dialysis for kidney failure, mechanical ventilation for respiratory failure, etc.). Commonly what is used when initially sepsis is diagnosed is Vancomycin with other antibiotics like Imipenum, Cefepime, and others depending on what the blood culture shows as the microorganism if SIRS is caused by a bacterial infection (many times it is).